Next: Specific Heat Capacity Up: Ideal Gas Previous: Ideal Gas Law
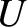 be the internal energy of an ideal gas. Internal energy is the energy that the gas possesses
by virtue of the random motions of its constituent molecules. Consider a process by which an
infinitesimal amount of heat,
be the internal energy of an ideal gas. Internal energy is the energy that the gas possesses
by virtue of the random motions of its constituent molecules. Consider a process by which an
infinitesimal amount of heat, 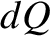 , is absorbed by the gas, and an infinitesimal amount of work,
, is absorbed by the gas, and an infinitesimal amount of work, 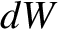 ,
is performed on the gas. According to the first law of thermodynamics, which is a statement of
energy conservation that was first explicitly formulated by Rudolf Clausius in 1850,
In reality,
,
is performed on the gas. According to the first law of thermodynamics, which is a statement of
energy conservation that was first explicitly formulated by Rudolf Clausius in 1850,
In reality, 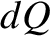 cannot be directly measured, and is, instead, inferred to be the difference between the change in the
gas's internal energy and the work performed on the gas, both of which can be directly measured, according to the previous equation.
cannot be directly measured, and is, instead, inferred to be the difference between the change in the
gas's internal energy and the work performed on the gas, both of which can be directly measured, according to the previous equation.
Consider an ideal gas in a cylindrical container of cross-sectional area  . Suppose that the top of the
container is a movable piston, and that the gas pushes the piston upward a distance
. Suppose that the top of the
container is a movable piston, and that the gas pushes the piston upward a distance 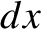 . Now, from the
definition of pressure, the gas exerts a force
. Now, from the
definition of pressure, the gas exerts a force 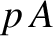 on the piston. Thus, the gas does work
on the piston. Thus, the gas does work 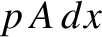 on the piston. (See Section 1.3.2.) Hence, the work done on the gas is
on the piston. (See Section 1.3.2.) Hence, the work done on the gas is
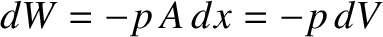 , where
, where
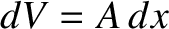 is the change in volume of the gas. This is a general result.
Hence, Equation (5.101) becomes
is the change in volume of the gas. This is a general result.
Hence, Equation (5.101) becomes