Next: First Law of Thermodynamics Up: Ideal Gas Previous: Ideal Gas
According to Boyle's law, which is an experimental result that was first reported by Robert Boyle in 1660, the pressure of an ideal gas is inversely proportional to its volume, at fixed temperature. According to Charles's law, which is another experimental result that was obtained by Jacques Charles in 1787, the volume of an ideal gas is proportional to its absolute temperature, at fixed pressure. Finally, according to Avogadro's law, which was first proposed by Amedeo Avogadro in 1812, equal volumes of all ideal gases, at the same temperature and pressure, contain the same number of molecules. These three laws imply that an ideal gas is governed by the following equation of state:
Here,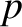 is the gas pressure,
is the gas pressure, 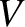 the volume,
the volume, 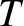 the absolute temperature,
the absolute temperature, 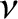 the number of
moles of molecules in the gas, and
the number of
moles of molecules in the gas, and
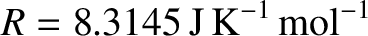 |
(5.98) |
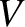 and
and 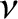 are extensive quantities. That is, if we double the size
of the system (by combining two identical systems) then we double the values of these quantities. On the other hand,
are extensive quantities. That is, if we double the size
of the system (by combining two identical systems) then we double the values of these quantities. On the other hand, 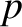 and
and 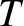 are intensive
quantities. That is, if we double the size of the system then the values of these quantities are left unchanged.
are intensive
quantities. That is, if we double the size of the system then the values of these quantities are left unchanged.
Absolute temperature is measured in degrees kelvin (K) on a scale in which
absolute zero (i.e., the lowest possible temperature) is
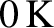 , and the
triple point of water (i.e., the unique temperature at which all three phases of water coexist) is
, and the
triple point of water (i.e., the unique temperature at which all three phases of water coexist) is
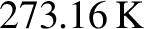 .
.
One mole of molecules contains Avogadro's number of molecules; that is,
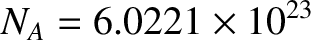 |
(5.99) |
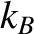 , is defined
, is defined