Next: Wave Mechanics Up: Experimental Basis of Quantum Previous: Two-Source Particle Interference
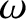 , and wavenumber,
, and wavenumber, 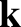 , of a particle wave is related to the
energy,
, of a particle wave is related to the
energy, 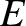 , and momentum,
, and momentum, 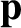 , of its constituent particles according to
Obviously, these relations are the same as the relations between the angular frequency and wavenumber
of an electromagnetic wave and the energy and momentum of its constituent photons. (See Section 4.1.2.)
As discussed in Sections 4.1.6 and 4.1.7, relation (4.9) can be verified experimentally.
On the other hand, relation (4.8) is adopted on the basis of an analogy drawn between massive particles and photons.
, of its constituent particles according to
Obviously, these relations are the same as the relations between the angular frequency and wavenumber
of an electromagnetic wave and the energy and momentum of its constituent photons. (See Section 4.1.2.)
As discussed in Sections 4.1.6 and 4.1.7, relation (4.9) can be verified experimentally.
On the other hand, relation (4.8) is adopted on the basis of an analogy drawn between massive particles and photons.