Next: Photoelectric Effect Up: Experimental Basis of Quantum Previous: Experimental Basis of Quantum
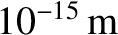 , whereas
electrons act like point particles (i.e., they have no discernible spatial extent).
Waves, on the other hand, are non-localized in space. In fact, a wave is defined as a disturbance that
is periodic in space, with some finite periodicity length (i.e., wavelength). Hence, it is fairly meaningless to
talk about a disturbance being a wave unless it extends over
a region of space that is at least a few wavelengths in size.
, whereas
electrons act like point particles (i.e., they have no discernible spatial extent).
Waves, on the other hand, are non-localized in space. In fact, a wave is defined as a disturbance that
is periodic in space, with some finite periodicity length (i.e., wavelength). Hence, it is fairly meaningless to
talk about a disturbance being a wave unless it extends over
a region of space that is at least a few wavelengths in size.
The classical scenario, just described, in which particles and waves are distinct phenomena, had to be significantly modified in the early decades of the 20th century. During this time period, physicists discovered, much to their surprise, that, under certain circumstances, waves act as particles, and particles act as waves. This bizarre behavior is known as wave-particle duality. For instance, the photoelectric effect (see Section 4.1.2) shows that electromagnetic waves sometimes act like swarms of massless particles called photons. Moreover, the phenomenon of electron diffraction by atomic lattices (see Section 4.1.6) implies that electrons sometimes possess wave-like properties.
Wave-particle
duality usually only manifests itself on atomic and sub-atomic lengthscales (i.e., on lengthscales less than,
or of order,
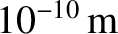 ; see Section 4.1.6.) The classical picture remains valid on significantly longer lengthscales. Thus,
on macroscopic lengthscales,
waves only act like waves, particles only act like particles, and there is no wave-particle duality. However, on
atomic lengthscales, classical mechanics, which governs the macroscopic behavior of massive particles, and
classical electrodynamics, which governs the macroscopic behavior of electromagnetic fields—neither of
which take wave-particle duality into account—must be replaced by new theories. The theories in question are called quantum mechanics and quantum electrodynamics,
respectively. In this section, we shall discuss a simple version of quantum mechanics in which the microscopic dynamics of
massive particles (i.e., particles with finite mass) is described
entirely in terms of wavefunctions. This
particular
version of quantum mechanics is known as wave mechanics. But, first, let us discuss the experimental evidence for
wave-particle duality in more detail.
; see Section 4.1.6.) The classical picture remains valid on significantly longer lengthscales. Thus,
on macroscopic lengthscales,
waves only act like waves, particles only act like particles, and there is no wave-particle duality. However, on
atomic lengthscales, classical mechanics, which governs the macroscopic behavior of massive particles, and
classical electrodynamics, which governs the macroscopic behavior of electromagnetic fields—neither of
which take wave-particle duality into account—must be replaced by new theories. The theories in question are called quantum mechanics and quantum electrodynamics,
respectively. In this section, we shall discuss a simple version of quantum mechanics in which the microscopic dynamics of
massive particles (i.e., particles with finite mass) is described
entirely in terms of wavefunctions. This
particular
version of quantum mechanics is known as wave mechanics. But, first, let us discuss the experimental evidence for
wave-particle duality in more detail.