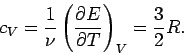Next: Isothermal and adiabatic expansion
Up: Classical thermodynamics
Previous: Heat capacity or specific
Now that we know the relationship between the specific heats at constant volume and
constant pressure for an ideal gas,
it would be nice if we could calculate either one of these quantities
from first principles. Classical thermodynamics cannot help us here. However,
it is quite easy to calculate the specific heat at constant volume using our
knowledge of statistical physics. Recall, that the variation of the number of
accessible states of an ideal gas with energy and volume is written
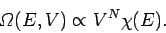 |
(301) |
For the specific case of a monatomic ideal gas, we worked out a more exact
expression for  in Sect. 3.8: i.e.,
in Sect. 3.8: i.e.,
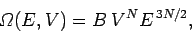 |
(302) |
where 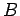 is a constant independent of the energy and volume. It follows that
is a constant independent of the energy and volume. It follows that
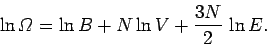 |
(303) |
The temperature is given by
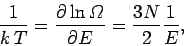 |
(304) |
so
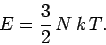 |
(305) |
Since, 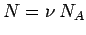 , and
, and 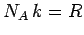 , we can rewrite the above expression as
, we can rewrite the above expression as
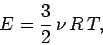 |
(306) |
where
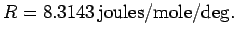 is the ideal gas constant. The above
formula tells us exactly how the internal energy of a monatomic ideal gas
depends on its temperature.
is the ideal gas constant. The above
formula tells us exactly how the internal energy of a monatomic ideal gas
depends on its temperature.
The molar specific heat at constant volume of a monatomic ideal gas is
clearly
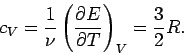 |
(307) |
This has the numerical value
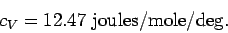 |
(308) |
Furthermore, we have
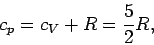 |
(309) |
and
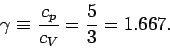 |
(310) |
We can see from the previous table that these predictions are borne out pretty
well for the monatomic gases Helium and Argon. Note that the specific heats of
polyatomic gases are larger than those of monatomic gases. This is because
polyatomic molecules can rotate around their centres of mass, as well as translate,
so polyatomic gases can store energy in the rotational, as well as
the translational,
energy states of their constituent particles. We shall analyze this effect in
greater detail later on in this course.



Next: Isothermal and adiabatic expansion
Up: Classical thermodynamics
Previous: Heat capacity or specific
Richard Fitzpatrick
2006-02-02