


Next: General interaction between macrosystems
Up: Statistical thermodynamics
Previous: Temperature
Let us now examine a purely mechanical interaction between macrostates, where one
or more of the external parameters is modified, but there is no exchange of
heat energy. Consider, for the sake of simplicity, a situation where only
one external parameter 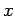 of the system is free to vary.
In general, the number of microstates
accessible to the system when the overall energy lies between
of the system is free to vary.
In general, the number of microstates
accessible to the system when the overall energy lies between  and
and 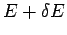 depends on the particular value of
depends on the particular value of 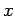 , so we can write
, so we can write
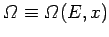 .
.
When 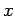 is changed by the amount
is changed by the amount 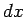 , the energy
, the energy 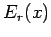 of a given
microstate
of a given
microstate
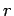 changes by
changes by
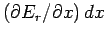 . The number of states
. The number of states
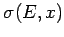 whose energy is changed from a value less than
whose energy is changed from a value less than  to a value
greater than
to a value
greater than  when the parameter changes from
when the parameter changes from 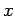 to
to 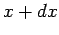 is given by
the number of microstates per unit energy range multiplied by the average
shift in energy of the microstates. Hence,
is given by
the number of microstates per unit energy range multiplied by the average
shift in energy of the microstates. Hence,
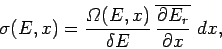 |
(190) |
where the mean value of
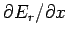 is taken over all accessible
microstates (i.e., all states where the energy lies between
is taken over all accessible
microstates (i.e., all states where the energy lies between  and
and 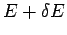 and
the external parameter takes the value
and
the external parameter takes the value 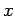 ). The above equation can also be written
). The above equation can also be written
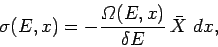 |
(191) |
where
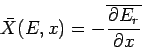 |
(192) |
is the mean generalized force conjugate to the external parameter 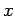 (see Sect. 4.4).
(see Sect. 4.4).
Consider the total number of microstates between  and
and 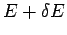 . When the
external parameter changes from
. When the
external parameter changes from 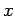 to
to 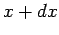 , the number of states in this energy
range changes by
, the number of states in this energy
range changes by
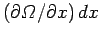 . This change is
due to the difference between the number of states which enter the
range because their energy is changed from a value less than
. This change is
due to the difference between the number of states which enter the
range because their energy is changed from a value less than  to one greater than
to one greater than
 and the number which
leave
because their energy is changed from a value less than
and the number which
leave
because their energy is changed from a value less than 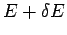 to one
greater than
to one
greater than 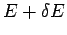 .
In symbols,
.
In symbols,
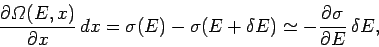 |
(193) |
which yields
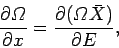 |
(194) |
where use has been made of Eq. (191). Dividing both sides by 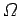 gives
gives
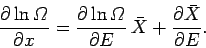 |
(195) |
However, according to the usual estimate
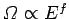 , the first term on the
right-hand side is of order
, the first term on the
right-hand side is of order
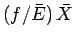 , whereas the second term is only
of order
, whereas the second term is only
of order
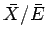 .
Clearly, for a macroscopic system with many degrees of freedom,
the second term is utterly negligible, so we have
.
Clearly, for a macroscopic system with many degrees of freedom,
the second term is utterly negligible, so we have
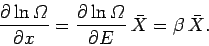 |
(196) |
When there are several external parameters
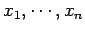 , so that
, so that
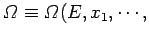
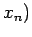 , the above derivation is valid for
each parameter
taken in isolation. Thus,
, the above derivation is valid for
each parameter
taken in isolation. Thus,
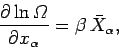 |
(197) |
where
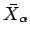 is the mean generalized force conjugate to the parameter
is the mean generalized force conjugate to the parameter
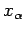 .
.



Next: General interaction between macrosystems
Up: Statistical thermodynamics
Previous: Temperature
Richard Fitzpatrick
2006-02-02
![]() is changed by the amount
is changed by the amount ![]() , the energy
, the energy ![]() of a given
microstate
of a given
microstate
![]() changes by
changes by
![]() . The number of states
. The number of states
![]() whose energy is changed from a value less than
whose energy is changed from a value less than ![]() to a value
greater than
to a value
greater than ![]() when the parameter changes from
when the parameter changes from ![]() to
to ![]() is given by
the number of microstates per unit energy range multiplied by the average
shift in energy of the microstates. Hence,
is given by
the number of microstates per unit energy range multiplied by the average
shift in energy of the microstates. Hence,
![]() and
and ![]() . When the
external parameter changes from
. When the
external parameter changes from ![]() to
to ![]() , the number of states in this energy
range changes by
, the number of states in this energy
range changes by
![]() . This change is
due to the difference between the number of states which enter the
range because their energy is changed from a value less than
. This change is
due to the difference between the number of states which enter the
range because their energy is changed from a value less than ![]() to one greater than
to one greater than
![]() and the number which
leave
because their energy is changed from a value less than
and the number which
leave
because their energy is changed from a value less than ![]() to one
greater than
to one
greater than ![]() .
In symbols,
.
In symbols,
![]() , so that
, so that
![]()
![]() , the above derivation is valid for
each parameter
taken in isolation. Thus,
, the above derivation is valid for
each parameter
taken in isolation. Thus,