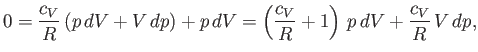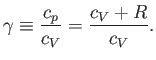Next: Hydrostatic Equilibrium of Atmosphere
Up: Classical Thermodynamics
Previous: Calculation of Specific Heats
Suppose that the temperature of an ideal
gas is held constant by keeping the gas in thermal
contact with a heat reservoir. If the gas is allowed to expand quasi-statically
under these so-called isothermal conditions then the ideal gas equation of state
tells us that
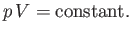 |
(6.52) |
This result is known as the isothermal gas law.
Suppose, now, that the gas is thermally isolated from its surroundings. If
the gas is allowed to expand quasi-statically under these so-called
adiabatic
conditions then
it does work on its environment, and, hence, its internal energy is reduced,
and its temperature changes. Let us calculate the relationship between the
pressure and volume of the gas during adiabatic expansion.
According to the first law of thermodynamics,
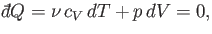 |
(6.53) |
in an adiabatic process (in which no heat is absorbed). [See Equation (6.35).] The ideal gas
equation
of state, (6.10), can be differentiated, yielding
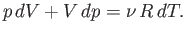 |
(6.54) |
The temperature increment, 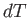 , can be eliminated between the previous two expressions
to give
, can be eliminated between the previous two expressions
to give
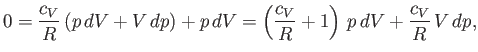 |
(6.55) |
which reduces to
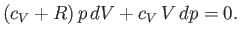 |
(6.56) |
Dividing through by
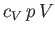 yields
yields
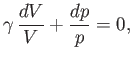 |
(6.57) |
where
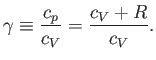 |
(6.58) |
It turns out that 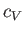 is a slowly-varying function of temperature in most
gases. Consequently, it is usually a good approximation to treat the ratio
of specific heats,
is a slowly-varying function of temperature in most
gases. Consequently, it is usually a good approximation to treat the ratio
of specific heats, 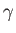 , as a constant, at least over a limited temperature
range. If
, as a constant, at least over a limited temperature
range. If 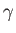 is constant then we can integrate Equation (6.57) to give
is constant then we can integrate Equation (6.57) to give
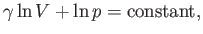 |
(6.59) |
or
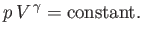 |
(6.60) |
This result is known as the adiabatic gas law.
It is straightforward to obtain analogous relationships between  and
and  , and between
, and between  and
and  ,
during adiabatic expansion or contraction. In fact, because
,
during adiabatic expansion or contraction. In fact, because
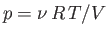 , the previous formula
also implies that
, the previous formula
also implies that
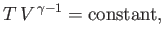 |
(6.61) |
and
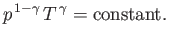 |
(6.62) |
Equations (6.60)-(6.62) are all completely equivalent.



Next: Hydrostatic Equilibrium of Atmosphere
Up: Classical Thermodynamics
Previous: Calculation of Specific Heats
Richard Fitzpatrick
2016-01-25