Consider a neutral gas consisting of identical hard-sphere molecules of
mass ![]() and
diameter
and
diameter ![]() . Admittedly, this is not a particularly
physical model of a neutral gas,
but we are only considering it for illustrative purposes. The fluid
equations for such a gas are similar to Equations (4.47)-(4.49):
. Admittedly, this is not a particularly
physical model of a neutral gas,
but we are only considering it for illustrative purposes. The fluid
equations for such a gas are similar to Equations (4.47)-(4.49):
The mean-free-path, ![]() , for hard-sphere molecules is given by
, for hard-sphere molecules is given by
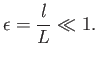 |
(4.59) |
In the Chapman-Enskog scheme, the distribution function is expanded, order by order,
in the small parameter ![]() :
:
| (4.60) |
![$\displaystyle f_0({\bf r}, {\bf v}, t) = n({\bf r}) \left[\frac{m}{2\pi\,T({\bf...
...ght]^{3/2}\,\exp\!\left[-\frac{m\,({\bf v}- {\bf V})^2} {2\,T({\bf r})}\right].$](img1113.png) |
(4.61) |
It is possible to linearize the kinetic equation, and then rearrange
it so as to obtain an integral equation for ![]() in terms of
in terms of ![]() .
This rearrangement crucially depends on the bilinearity of the collision
operator.
Incidentally, the equation is integral because the collision operator is an integral
operator. The integral equation is solved by expanding
.
This rearrangement crucially depends on the bilinearity of the collision
operator.
Incidentally, the equation is integral because the collision operator is an integral
operator. The integral equation is solved by expanding ![]() in velocity space
using Laguerre polynomials (sometimes called Sonine polynomials). It is
possible to reduce the integral equation to an infinite set of simultaneous
algebraic equations for the coefficients in this expansion. If the expansion
is truncated, after
in velocity space
using Laguerre polynomials (sometimes called Sonine polynomials). It is
possible to reduce the integral equation to an infinite set of simultaneous
algebraic equations for the coefficients in this expansion. If the expansion
is truncated, after ![]() terms, say, then these algebraic equations can be solved for
the coefficients. It turns out that the Laguerre polynomial expansion
converges very rapidly. Thus, it is conventional to keep only the first two
terms in this expansion, which is usually sufficient to ensure an accuracy of
about
terms, say, then these algebraic equations can be solved for
the coefficients. It turns out that the Laguerre polynomial expansion
converges very rapidly. Thus, it is conventional to keep only the first two
terms in this expansion, which is usually sufficient to ensure an accuracy of
about ![]() percent in the final result. Finally, the appropriate moments
of
percent in the final result. Finally, the appropriate moments
of ![]() are taken, so as to obtain expression for the heat flux density
and the viscosity
tensor. Strictly speaking, after evaluating
are taken, so as to obtain expression for the heat flux density
and the viscosity
tensor. Strictly speaking, after evaluating ![]() , we should then go on to
evaluate
, we should then go on to
evaluate ![]() , so as to ensure that
, so as to ensure that ![]() really is negligible compared to
really is negligible compared to ![]() .
In reality, this is never done because the mathematical difficulties involved
in such a calculation are prohibitive.
.
In reality, this is never done because the mathematical difficulties involved
in such a calculation are prohibitive.
The Chapman-Enskog method outlined previously can be applied to any assumed force law between molecules, provided that the force is sufficiently short-range (i.e., provided that it falls off faster with increasing separation than the Coulomb force). For all sensible force laws, the viscosity tensor is given by
| (4.64) | ||
| (4.65) |
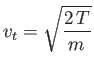 |
(4.69) |
Equations (4.66) and (4.67) have a simple physical interpretation. The viscous and thermal
diffusivities of a neutral gas can be accounted for in terms of the
random-walk diffusion of molecules with excess momentum and energy, respectively.
Recall the standard result in stochastic theory that if particles
jump an average distance ![]() , in a random direction,
, in a random direction, ![]() times a second, then
the diffusivity associated with such motion is
times a second, then
the diffusivity associated with such motion is
![]() (Reif 1965).
Chapman-Enskog theory basically allows us to calculate the numerical constants
(Reif 1965).
Chapman-Enskog theory basically allows us to calculate the numerical constants
![]() and
and ![]() ,
multiplying
,
multiplying
![]() in the expressions for
in the expressions for ![]() and
and ![]() ,
for a given force law between molecules.
Obviously, these coefficients are different for different force laws. The
expression for the
mean-free-path,
,
for a given force law between molecules.
Obviously, these coefficients are different for different force laws. The
expression for the
mean-free-path, ![]() , is also different for different force laws.
, is also different for different force laws.