Next: Coulomb's Law Up: Electrostatic Fields Previous: Electrostatic Fields
In Elizabethan times, the physician William Gilbert coined the word “electric” (from the Greek word for amber) to describe the previously mentioned effect. It was later found that many materials become electric when rubbed with certain other materials. In 1733 (CE), the chemist Charles du Fay discovered that there are, in fact, two different types of electricity. When amber is rubbed with fur it acquires so-called “resinous” electricity. On the other hand, when glass is rubbed with silk it acquires so-called “vitreous” electricity. Electricity repels electricity of the same kind, but attracts electricity of the opposite kind. At the time, it was thought that electricity was created by friction.
Scientists in the 18th century eventually developed the concept of electric charge in order to account for a large body of observations made in countless electrical experiments. There are two types of electric charge; positive (which is equivalent to vitreous), and negative (which is equivalent to resinous). Like electric charges repel one another, whereas opposite charges attract. When two bodies are rubbed together, electric charge can be transferred from one to the other, but the total charge remains constant. Thus, when amber is rubbed with fur, there is transfer of electric charge such that the amber acquires a negative charge, and the fur an equal positive charge. Likewise, when glass is rubbed with silk, the glass acquires a positive charge, and the silk an equal negative charge. The idea that electric charge is a conserved quantity is attributed to the Benjamin Franklin (who is also to blame for the unfortunate sign convention that led to electrons having a negative charge).
In the 20th century, scientists, such as J.J. Thompson and Ernest Rutherford, discovered that the atoms out of which ordinary matter is composed consist of two components; a relatively massive, positively charged nucleus, surrounded by a cloud of relatively light, negatively charged particles called electrons. Electrons and atomic nuclei carry fixed electrical charges, and are essentially indestructible (provided that we neglect nuclear reactions). Under normal circumstances, only the electrons are mobile. Thus, when amber is rubbed with fur, electrons are transferred from the fur to the amber, giving the amber an excess of electrons, and, hence, a negative electric charge, and the fur a deficit of electrons, and, hence, a positive charge. Substances normally contain neither an excess nor a deficit of electrons, and are, therefore, electrically neutral.
The SI unit of electric charge is the coulomb (C). The electric charge of an electron is
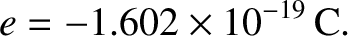 |
(2.1) |