Next: Thermal Expansion Up: Applications of Statistical Mechanics Previous: Spin-1/2 Paramagnetism
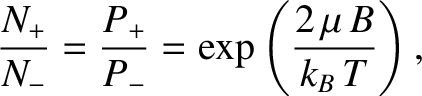 |
(5.351) |
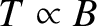 . This is known
as the magnetocaloric effect.
. This is known
as the magnetocaloric effect.
The magnetocaloric effect is the basis of a method of cooling atomic systems down to very low
temperatures that is known as adiabatic demagnetization. In this scheme, the
sample is initially in thermal contact with liquid helium at 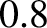 K. The sample is then magnetized. In the process,
heat is given off by the sample, and is conducted away by the liquid helium. Next, the sample
is thermally isolated by pumping out the liquid helium. Finally, the sample is demagnetized, leading to a reduction in its temperature via the magnetocaloric effect. Temperatures as low as
K. The sample is then magnetized. In the process,
heat is given off by the sample, and is conducted away by the liquid helium. Next, the sample
is thermally isolated by pumping out the liquid helium. Finally, the sample is demagnetized, leading to a reduction in its temperature via the magnetocaloric effect. Temperatures as low as 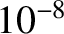 K have been
achieved by this method.
K have been
achieved by this method.