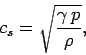Next: Calculation of specific heats
Up: Classical thermodynamics
Previous: The equation of state
Suppose that a body absorbs an amount of heat
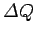 and its temperature consequently rises by
and its temperature consequently rises by
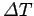 . The usual definition
of the heat capacity, or specific heat, of the body is
. The usual definition
of the heat capacity, or specific heat, of the body is
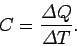 |
(285) |
If the body consists of 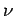 moles of some substance then the molar
specific heat (i.e., the specific heat of one mole of this substance ) is
defined
moles of some substance then the molar
specific heat (i.e., the specific heat of one mole of this substance ) is
defined
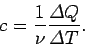 |
(286) |
In writing the above expressions, we have tacitly assumed that the specific heat
of a body is independent of its temperature. In general, this is not true. We
can overcome this problem by only allowing the body in question to absorb a very
small amount of heat, so that its temperature only rises slightly, and its
specific heat remains approximately constant. In the limit as the amount of
absorbed heat becomes infinitesimal, we obtain
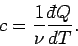 |
(287) |
In classical thermodynamics, it is usual to define two specific heats. Firstly,
the molar specific heat at constant volume, denoted
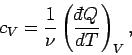 |
(288) |
and, secondly, the molar specific heat at constant pressure, denoted
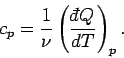 |
(289) |
Consider the molar specific heat at constant volume of an ideal gas.
Since 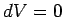 , no work is done by
the gas,
and the first law of thermodynamics reduces to
, no work is done by
the gas,
and the first law of thermodynamics reduces to
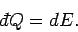 |
(290) |
It follows from Eq. (288) that
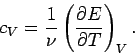 |
(291) |
Now, for an ideal gas the internal energy is volume independent.
Thus, the above expression implies that the specific heat at constant volume is also
volume independent. Since  is a function only of
is a function only of 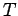 , we can write
, we can write
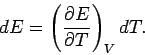 |
(292) |
The previous two expressions can be combined to give
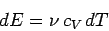 |
(293) |
for an ideal gas.
Let us now consider the molar specific heat at constant pressure of an ideal
gas. In general, if the
pressure is kept constant then the volume changes, and so the gas does work on its
environment. According to the first law of thermodynamics,
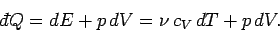 |
(294) |
The equation of state of an ideal gas tells us that if the
volume changes by 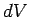 , the temperature changes by
, the temperature changes by 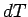 , and the pressure
remains constant, then
, and the pressure
remains constant, then
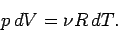 |
(295) |
The previous two equations can be combined to give
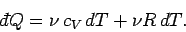 |
(296) |
Now, by definition
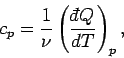 |
(297) |
so we obtain
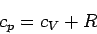 |
(298) |
for an ideal gas. This is a very famous result. Note that at constant volume
all of the heat absorbed by the gas goes into increasing its internal energy,
and, hence, its temperature, whereas at constant pressure some of the absorbed
heat is used to do work on the environment as the volume increases. This
means that, in the latter case,
less heat is available to increase the temperature of the gas.
Thus, we expect the specific heat at constant pressure to exceed that at
constant volume, as indicated by the above formula.
The ratio of the two specific heats 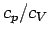 is conventionally denoted
is conventionally denoted
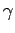 . We have
. We have
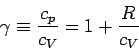 |
(299) |
for an ideal gas. In fact, 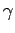 is very easy to measure because the speed
of sound in an ideal gas is written
is very easy to measure because the speed
of sound in an ideal gas is written
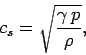 |
(300) |
where 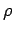 is the density. Table 2
lists some experimental measurements
of
is the density. Table 2
lists some experimental measurements
of 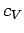 and
and 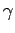 for common gases. The extent of the agreement between
for common gases. The extent of the agreement between 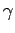 calculated from Eq. (299) and the experimental
calculated from Eq. (299) and the experimental 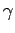 is quite remarkable.
is quite remarkable.
Table 2:
Specific heats of common gases in joules/mole/deg. (at 15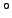 C and 1
atm.) From Reif.
C and 1
atm.) From Reif.
| Gas |
Symbol |
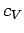 |
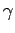 |
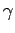 |
| |
|
(experiment) |
(experiment) |
(theory) |
| Helium |
He |
12.5 |
1.666 |
1.666 |
| Argon |
Ar |
12.5 |
1.666 |
1.666 |
| Nitrogen |
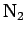 |
20.6 |
1.405 |
1.407 |
| Oxygen |
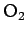 |
21.1 |
1.396 |
1.397 |
| Carbon Dioxide |
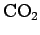 |
28.2 |
1.302 |
1.298 |
| Ethane |
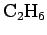 |
39.3 |
1.220 |
1.214 |
|



Next: Calculation of specific heats
Up: Classical thermodynamics
Previous: The equation of state
Richard Fitzpatrick
2006-02-02
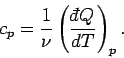
![]() , no work is done by
the gas,
and the first law of thermodynamics reduces to
, no work is done by
the gas,
and the first law of thermodynamics reduces to
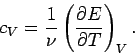
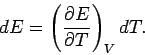
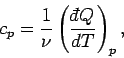
![]() is conventionally denoted
is conventionally denoted
![]() . We have
. We have