


Next: Thermodynamics and statistical thermodynamics
Up: Introduction
Previous: The need for a
It is useful, at this stage,
to make a distinction between the different sizes of the systems
that we are going to examine. We shall call a system microscopic if it is
roughly of atomic dimensions, or smaller. On the other hand, we shall call
a system macroscopic when it is large enough to be visible in the
ordinary sense. This is a rather inexact definition. The exact definition
depends on the number of particles in the system, which we shall call 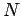 .
A system is macroscopic if
.
A system is macroscopic if
which means that statistical arguments can be applied to reasonable accuracy.
For instance, if we wish to keep the statistical error below
one percent then a macroscopic system would have to contain more than about
ten thousand particles. Any system containing less than this number of
particles would be regarded as essentially microscopic, and, hence,
statistical arguments could not be applied to such a system without
unacceptable error.
Richard Fitzpatrick
2006-02-02