

Next: Heat engines
Up: Classical thermodynamics
Previous: The isothermal atmosphere
Of course, we know that the atmosphere is not isothermal. In fact, air
temperature falls quite noticeably with
increasing altitude. In ski resorts, you are told to
expect the temperature to drop by about 1 degree per 100 meters you go upwards.
Many people cannot understand
why the atmosphere gets colder the higher up you go. They reason that as higher altitudes
are closer to the Sun they ought to be hotter.
In fact, the explanation is quite
simple. It depends on three important properties of air. The first important
property is that air is transparent to most, but by no means all, of the
electromagnetic spectrum. In particular, most infrared radiation, which carries heat
energy, passes straight through the lower atmosphere and heats the ground. In other
words, the lower atmosphere is heated from below, not from above. The
second important
property of air is that it is constantly in motion. In fact, the lower 20 kilometers
of the atmosphere (the so called troposphere)
are fairly thoroughly mixed. You might think that this
would imply that the atmosphere is
isothermal. However, this is not the case because of
the final important properly of air: i.e., it is a very poor conductor of heat.
This, of course, is why woolly sweaters work: they trap a layer of air close to
the body, and because air is such a poor conductor of heat you stay warm.
Imagine a packet of air which is being swirled around in the atmosphere. We would
expect it to always remain at the same pressure as its surroundings, otherwise it
would be mechanically unstable. It is also plausible that the packet moves around
too quickly to effectively exchange heat with its surroundings, since
air is very a poor heat conductor, and heat flow is consequently quite a
slow process. So,
to a first approximation, the air in the packet is adiabatic.
In a steady-state atmosphere, we expect that as the packet moves upwards,
expands due to the reduced pressure, and cools adiabatically, its temperature
always remains the same as that of its immediate surroundings.
This means that we
can use the adiabatic gas law to characterize the cooling of the
atmosphere with increasing altitude. In this particular
case, the most useful manifestation of the adiabatic law is
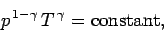 |
(329) |
giving
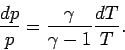 |
(330) |
Combining the above relation with the equation of hydrostatic equilibrium,
(326), we obtain
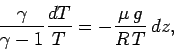 |
(331) |
or
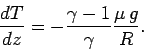 |
(332) |
Now, the ratio of specific heats for air (which is effectively
a diatomic gas) is about 1.4 (see Tab. 2).
Hence, we can calculate, from the
above expression, that the temperature of the atmosphere decreases with
increasing height
at a constant rate of 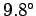 centigrade per kilometer.
This value is called the adiabatic lapse rate of the atmosphere.
Our calculation accords well with the
``
centigrade per kilometer.
This value is called the adiabatic lapse rate of the atmosphere.
Our calculation accords well with the
``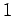 degree colder per 100 meters higher'' rule of thumb used in ski resorts.
The basic reason why air is colder at higher altitudes is
that it expands as its pressure decreases with height. It, therefore, does work
on its environment, without absorbing any heat (because of its low thermal
conductivity),
so its internal energy, and, hence, its temperature decreases.
degree colder per 100 meters higher'' rule of thumb used in ski resorts.
The basic reason why air is colder at higher altitudes is
that it expands as its pressure decreases with height. It, therefore, does work
on its environment, without absorbing any heat (because of its low thermal
conductivity),
so its internal energy, and, hence, its temperature decreases.
According to the adiabatic lapse rate calculated above, the air temperature at
the cruising altitude of airliners (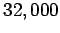 feet) should be about
feet) should be about 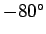 centigrade (assuming a sea level temperature of
centigrade (assuming a sea level temperature of 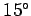 centigrade).
In fact, this is somewhat of an underestimate. A more realistic value is about
centigrade).
In fact, this is somewhat of an underestimate. A more realistic value is about
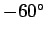 centigrade.
The explanation for this
discrepancy is the presence of
water vapour in the atmosphere. As air rises, expands, and cools, water
vapour condenses out releasing latent heat which prevents the temperature
from falling as rapidly with height as the adiabatic lapse rate would indicate.
In fact, in the Tropics, where the humidity is very high, the lapse rate of
the atmosphere (i.e., the rate of decrease of temperature with altitude)
is significantly less than the adiabatic value. The adiabatic
lapse rate is only observed when the humidity is low. This is the case in deserts,
in the Arctic (where water vapour is frozen out of the atmosphere), and, of course,
in ski resorts.
centigrade.
The explanation for this
discrepancy is the presence of
water vapour in the atmosphere. As air rises, expands, and cools, water
vapour condenses out releasing latent heat which prevents the temperature
from falling as rapidly with height as the adiabatic lapse rate would indicate.
In fact, in the Tropics, where the humidity is very high, the lapse rate of
the atmosphere (i.e., the rate of decrease of temperature with altitude)
is significantly less than the adiabatic value. The adiabatic
lapse rate is only observed when the humidity is low. This is the case in deserts,
in the Arctic (where water vapour is frozen out of the atmosphere), and, of course,
in ski resorts.
Suppose that the lapse rate of the atmosphere differs from the adiabatic value.
Let us ignore the complication of water vapour and assume that the atmosphere
is dry. Consider a packet of air which moves slightly upwards
from its equilibrium height. The temperature of the packet will
decrease with altitude according to the adiabatic lapse rate, because its
expansion is adiabatic. We assume that the packet always maintains pressure
balance with its surroundings. It follows that since
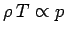 ,
according to the ideal gas law, then
,
according to the ideal gas law, then
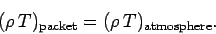 |
(333) |
If the atmospheric lapse rate is less than the adiabatic value then
 implying that
implying that
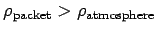 . So, the packet will be denser than its immediate
surroundings, and will, therefore, tend to fall back to its original height.
Clearly, an atmosphere whose lapse rate is less than the adiabatic value is
stable. On the other hand, if the atmospheric lapse rate exceeds the adiabatic
value then, after rising a little way,
the packet will be less dense than its immediate surroundings, and will, therefore,
continue to rise due to buoyancy effects.
Clearly, an atmosphere whose lapse rate is greater
than the adiabatic value is unstable. This effect is of great importance
in Meteorology. The normal stable state of the atmosphere is for the lapse rate
to be slightly less than
the adiabatic value. Occasionally, however, the lapse rate exceeds
the adiabatic value, and this is always associated with
extremely disturbed weather patterns.
. So, the packet will be denser than its immediate
surroundings, and will, therefore, tend to fall back to its original height.
Clearly, an atmosphere whose lapse rate is less than the adiabatic value is
stable. On the other hand, if the atmospheric lapse rate exceeds the adiabatic
value then, after rising a little way,
the packet will be less dense than its immediate surroundings, and will, therefore,
continue to rise due to buoyancy effects.
Clearly, an atmosphere whose lapse rate is greater
than the adiabatic value is unstable. This effect is of great importance
in Meteorology. The normal stable state of the atmosphere is for the lapse rate
to be slightly less than
the adiabatic value. Occasionally, however, the lapse rate exceeds
the adiabatic value, and this is always associated with
extremely disturbed weather patterns.
Let us consider the temperature, pressure, and density profiles in an
adiabatic atmosphere. We can directly integrate Eq. (332) to
give
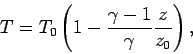 |
(334) |
where 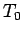 is the ground level temperature, and
is the ground level temperature, and
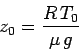 |
(335) |
is the isothermal scale-height calculated using this temperature. The
pressure profile is easily calculated from the adiabatic gas law
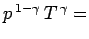 constant, or
constant, or
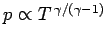 . It
follows that
. It
follows that
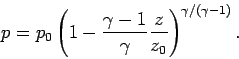 |
(336) |
Consider the limit
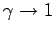 . In this limit, Eq. (334) yields
. In this limit, Eq. (334) yields
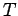 independent of height (i.e., the atmosphere becomes isothermal). We can evaluate
Eq. (336) in the limit
as
independent of height (i.e., the atmosphere becomes isothermal). We can evaluate
Eq. (336) in the limit
as
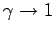 using the mathematical identity
using the mathematical identity
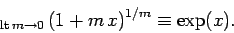 |
(337) |
We obtain
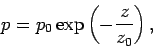 |
(338) |
which, not surprisingly, is the predicted pressure variation
in an isothermal atmosphere. In reality,
the ratio of specific heats of the atmosphere is not unity, it is about 1.4
(i.e., the ratio for diatomic gases), which
implies that in the real atmosphere
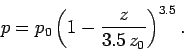 |
(339) |
In fact, this formula gives very similar results to the exponential formula,
Eq. (338), for heights below one scale-height (i.e., 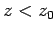 ). For heights
above one scale-height, the exponential formula tends to predict too low
a pressure. So, in an adiabatic atmosphere, the pressure falls off less quickly
with altitude than in an isothermal atmosphere, but this effect is only really
noticeable at pressures significantly below one atmosphere. In fact, the isothermal
formula is a pretty good approximation below altitudes of about 10 kilometers.
Since
). For heights
above one scale-height, the exponential formula tends to predict too low
a pressure. So, in an adiabatic atmosphere, the pressure falls off less quickly
with altitude than in an isothermal atmosphere, but this effect is only really
noticeable at pressures significantly below one atmosphere. In fact, the isothermal
formula is a pretty good approximation below altitudes of about 10 kilometers.
Since
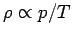 , the variation of density with height goes like
, the variation of density with height goes like
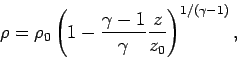 |
(340) |
where 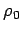 is the density at ground level. Thus, the density falls off
more rapidly with altitude than the temperature, but less rapidly than the
pressure.
is the density at ground level. Thus, the density falls off
more rapidly with altitude than the temperature, but less rapidly than the
pressure.
Note that an adiabatic atmosphere has a sharp upper boundary. Above height
![$z_1 = [\gamma/(\gamma-1)]\,z_0$](img862.png) the temperature, pressure, and density are
all zero: i.e., there is no atmosphere. For real air, with
the temperature, pressure, and density are
all zero: i.e., there is no atmosphere. For real air, with 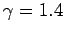 ,
,
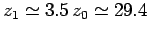 kilometers. This behaviour is quite different
to that of an isothermal atmosphere, which has a diffuse upper boundary. In reality,
there is no sharp upper boundary to the atmosphere. The adiabatic gas law
does not apply above about 20 kilometers (i.e., in the stratosphere) because
at these altitudes the air is no longer strongly mixed. Thus, in the stratosphere
the pressure falls off exponentially with increasing height.
kilometers. This behaviour is quite different
to that of an isothermal atmosphere, which has a diffuse upper boundary. In reality,
there is no sharp upper boundary to the atmosphere. The adiabatic gas law
does not apply above about 20 kilometers (i.e., in the stratosphere) because
at these altitudes the air is no longer strongly mixed. Thus, in the stratosphere
the pressure falls off exponentially with increasing height.
In conclusion, we have demonstrated that the temperature
of the lower atmosphere should fall off
approximately linearly with increasing height above ground level, whilst the
pressure should fall off far more rapidly than this, and the density should
fall off at some intermediate rate. We have also shown that the
lapse rate of the temperature should be about 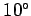 centigrade per kilometer
in dry air, but somewhat
less than this in wet air. In fact, all off these predictions
are, more or less, correct. It is amazing that such accurate predictions can
be obtained from the two simple laws,
centigrade per kilometer
in dry air, but somewhat
less than this in wet air. In fact, all off these predictions
are, more or less, correct. It is amazing that such accurate predictions can
be obtained from the two simple laws, 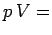 constant for an isothermal gas, and
constant for an isothermal gas, and
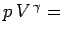 constant for an adiabatic gas.
constant for an adiabatic gas.


Next: Heat engines
Up: Classical thermodynamics
Previous: The isothermal atmosphere
Richard Fitzpatrick
2006-02-02
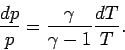
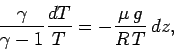
![]() feet) should be about
feet) should be about ![]() centigrade (assuming a sea level temperature of
centigrade (assuming a sea level temperature of ![]() centigrade).
In fact, this is somewhat of an underestimate. A more realistic value is about
centigrade).
In fact, this is somewhat of an underestimate. A more realistic value is about
![]() centigrade.
The explanation for this
discrepancy is the presence of
water vapour in the atmosphere. As air rises, expands, and cools, water
vapour condenses out releasing latent heat which prevents the temperature
from falling as rapidly with height as the adiabatic lapse rate would indicate.
In fact, in the Tropics, where the humidity is very high, the lapse rate of
the atmosphere (i.e., the rate of decrease of temperature with altitude)
is significantly less than the adiabatic value. The adiabatic
lapse rate is only observed when the humidity is low. This is the case in deserts,
in the Arctic (where water vapour is frozen out of the atmosphere), and, of course,
in ski resorts.
centigrade.
The explanation for this
discrepancy is the presence of
water vapour in the atmosphere. As air rises, expands, and cools, water
vapour condenses out releasing latent heat which prevents the temperature
from falling as rapidly with height as the adiabatic lapse rate would indicate.
In fact, in the Tropics, where the humidity is very high, the lapse rate of
the atmosphere (i.e., the rate of decrease of temperature with altitude)
is significantly less than the adiabatic value. The adiabatic
lapse rate is only observed when the humidity is low. This is the case in deserts,
in the Arctic (where water vapour is frozen out of the atmosphere), and, of course,
in ski resorts.
![]() ,
according to the ideal gas law, then
,
according to the ideal gas law, then
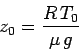
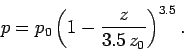
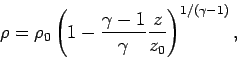
![]() the temperature, pressure, and density are
all zero: i.e., there is no atmosphere. For real air, with
the temperature, pressure, and density are
all zero: i.e., there is no atmosphere. For real air, with ![]() ,
,
![]() kilometers. This behaviour is quite different
to that of an isothermal atmosphere, which has a diffuse upper boundary. In reality,
there is no sharp upper boundary to the atmosphere. The adiabatic gas law
does not apply above about 20 kilometers (i.e., in the stratosphere) because
at these altitudes the air is no longer strongly mixed. Thus, in the stratosphere
the pressure falls off exponentially with increasing height.
kilometers. This behaviour is quite different
to that of an isothermal atmosphere, which has a diffuse upper boundary. In reality,
there is no sharp upper boundary to the atmosphere. The adiabatic gas law
does not apply above about 20 kilometers (i.e., in the stratosphere) because
at these altitudes the air is no longer strongly mixed. Thus, in the stratosphere
the pressure falls off exponentially with increasing height.
![]() centigrade per kilometer
in dry air, but somewhat
less than this in wet air. In fact, all off these predictions
are, more or less, correct. It is amazing that such accurate predictions can
be obtained from the two simple laws,
centigrade per kilometer
in dry air, but somewhat
less than this in wet air. In fact, all off these predictions
are, more or less, correct. It is amazing that such accurate predictions can
be obtained from the two simple laws, ![]() constant for an isothermal gas, and
constant for an isothermal gas, and
![]() constant for an adiabatic gas.
constant for an adiabatic gas.