

Next: Quantum Theory of Light
Up: Wave-Particle Duality
Previous: Classical Light Waves
Photoelectric Effect
The so-called photoelectric effect, by which a polished metal surface emits electrons
when illuminated by visible and ultra-violet light, was discovered by Heinrich Hertz in 1887.
The following facts regarding this effect can be established via careful
observation. First, a given surface only emits electrons when the frequency
of the light with which it is illuminated exceeds a certain threshold value,
which is a property of the metal. Second, the current of photoelectrons, when it
exists, is proportional to the intensity of the light falling on the surface.
Third, the energy of the photoelectrons is independent of the light intensity,
but varies linearly with the light frequency. These facts are
inexplicable within the framework of classical physics.
In 1905, Albert Einstein proposed a radical new theory of light in order to
account for the photoelectric effect. According to this theory, light
of fixed frequency 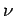 consists of a collection of indivisible discrete packages, called
quanta,
consists of a collection of indivisible discrete packages, called
quanta,![[*]](footnote.png) whose energy is
whose energy is
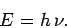 |
(58) |
Here,
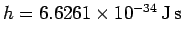 is a new constant of nature,
known as Planck's constant. Incidentally,
is a new constant of nature,
known as Planck's constant. Incidentally, 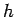 is called Planck's constant, rather than Einstein's constant, because Max Planck first introduced the concept of the quantization of light, in 1900, whilst trying
to account for the electromagnetic spectrum of a black body (i.e.,
a perfect emitter and absorber of electromagnetic radiation).
is called Planck's constant, rather than Einstein's constant, because Max Planck first introduced the concept of the quantization of light, in 1900, whilst trying
to account for the electromagnetic spectrum of a black body (i.e.,
a perfect emitter and absorber of electromagnetic radiation).
Suppose that the electrons at the surface of a metal lie in a potential well
of depth 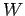 . In other words, the electrons have to acquire an energy
. In other words, the electrons have to acquire an energy 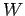 in order to be emitted from the surface. Here,
in order to be emitted from the surface. Here, 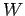 is generally called
the work function of the surface, and is a property of the
metal. Suppose that an electron absorbs a single quantum of light. Its energy
therefore increases by
is generally called
the work function of the surface, and is a property of the
metal. Suppose that an electron absorbs a single quantum of light. Its energy
therefore increases by 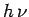 . If
. If 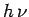 is greater than
is greater than 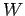 then the
electron is emitted from the surface with residual kinetic energy
then the
electron is emitted from the surface with residual kinetic energy
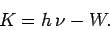 |
(59) |
Otherwise, the electron remains trapped in the potential well, and is not emitted. Here, we are assuming that the probability of an electron simultaneously absorbing
two or more light quanta is negligibly small compared to the probability of it
absorbing a single light quantum (as is, indeed, the case for
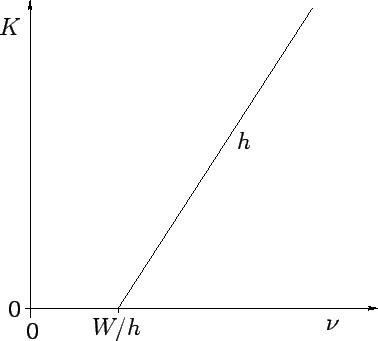
low intensity illumination). Incidentally, we can calculate Planck's
constant, and the work function of the metal, by simply plotting the kinetic
energy of the emitted photoelectrons as a function of the wave frequency,
as shown in Fig. 4. This plot is a straight-line whose slope is 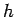 ,
and whose intercept with the
,
and whose intercept with the 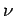 axis is
axis is 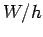 . Finally, the number
of emitted electrons increases with the intensity of the light because the
more intense the light the larger the flux of light quanta onto the surface.
Thus, Einstein's quantum theory is capable of accounting for all
three of the previously mentioned observational facts regarding the photoelectric
effect.
. Finally, the number
of emitted electrons increases with the intensity of the light because the
more intense the light the larger the flux of light quanta onto the surface.
Thus, Einstein's quantum theory is capable of accounting for all
three of the previously mentioned observational facts regarding the photoelectric
effect.
Figure 4:
Variation of the kinetic energy 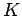 of photoelectrons with the wave-frequency
of photoelectrons with the wave-frequency 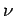 .
.
 |


Next: Quantum Theory of Light
Up: Wave-Particle Duality
Previous: Classical Light Waves
Richard Fitzpatrick
2010-07-20
![[*]](footnote.png) whose energy is
whose energy is
![]() consists of a collection of indivisible discrete packages, called
quanta,
consists of a collection of indivisible discrete packages, called
quanta,![[*]](footnote.png) whose energy is
whose energy is
![]() . In other words, the electrons have to acquire an energy
. In other words, the electrons have to acquire an energy ![]() in order to be emitted from the surface. Here,
in order to be emitted from the surface. Here, ![]() is generally called
the work function of the surface, and is a property of the
metal. Suppose that an electron absorbs a single quantum of light. Its energy
therefore increases by
is generally called
the work function of the surface, and is a property of the
metal. Suppose that an electron absorbs a single quantum of light. Its energy
therefore increases by ![]() . If
. If ![]() is greater than
is greater than ![]() then the
electron is emitted from the surface with residual kinetic energy
then the
electron is emitted from the surface with residual kinetic energy