


Next: Specific Heats
Up: Applications of Statistical Thermodynamics
Previous: Equipartition Theorem
Harmonic Oscillators
Our proof of the equipartition theorem depends crucially on the classical approximation. To see how
quantum effects modify this result, let us examine a particularly simple system
that we know how to analyze using both classical and quantum physics: namely,
a
simple harmonic oscillator. Consider a one-dimensional harmonic oscillator in equilibrium
with a heat reservoir held at absolute temperature  . The energy of the oscillator is given by
. The energy of the oscillator is given by
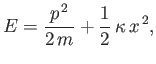 |
(7.136) |
where the first term on the right-hand side is the kinetic energy, involving the momentum,
 , and the mass,
, and the mass, 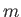 , and the second term is the potential energy, involving the displacement,
, and the second term is the potential energy, involving the displacement,
 , and the force constant,
, and the force constant, 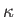 . Each of these terms is quadratic in the respective
variable. So, in the classical approximation, the equipartition theorem yields:
. Each of these terms is quadratic in the respective
variable. So, in the classical approximation, the equipartition theorem yields:
That is, the mean kinetic energy of the oscillator is equal
to the mean potential energy, which
equals
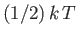 . It follows that the mean total energy is
. It follows that the mean total energy is
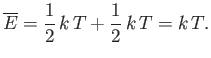 |
(7.139) |
According to quantum mechanics, the energy levels of a harmonic oscillator are equally
spaced, and satisfy
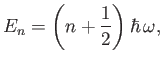 |
(7.140) |
where 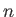 is a non-negative integer, and
is a non-negative integer, and
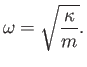 |
(7.141) |
(See Section C.11.)
The partition function for such an oscillator is given by
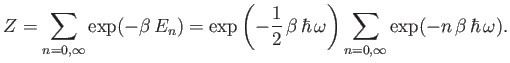 |
(7.142) |
Now,
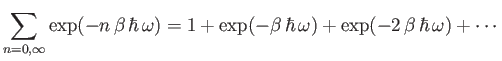 |
(7.143) |
is simply the sum of an infinite geometric series, and can be evaluated immediately to give
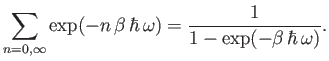 |
(7.144) |
(See Exercise 1.)
Thus, the partition function takes the form
![$\displaystyle Z = \frac{ \exp[-(1/2) \beta \hbar \omega]}{1-\exp(-\beta \hbar \omega)},$](img1522.png) |
(7.145) |
and
![$\displaystyle \ln Z = - \frac{1}{2} \beta \hbar \omega -\ln \left[1- \exp(-\beta \hbar \omega)\right].$](img1523.png) |
(7.146) |
The mean energy of the oscillator is given by [see Equation (7.35)]
![$\displaystyle \overline{E} = - \frac{\partial}{\partial \beta} \ln Z = - \left[...
...(-\beta \hbar \omega) \hbar \omega} {1-\exp(-\beta \hbar \omega)}\right],$](img1524.png) |
(7.147) |
or
![$\displaystyle \overline{E} = \hbar \omega \left[ \frac{1}{2} + \frac{1}{\exp( \beta \hbar \omega)-1} \right].$](img1525.png) |
(7.148) |
Consider the limit
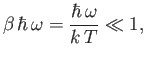 |
(7.149) |
in which the thermal energy, 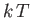 , is large compared to the separation,
, is large compared to the separation,
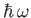 , between successive
energy levels. In this limit,
, between successive
energy levels. In this limit,
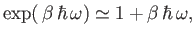 |
(7.150) |
so
![$\displaystyle \overline{E} \simeq \hbar \omega\left[\frac{1}{2} + \frac{1}{\be...
...omega}\right] \simeq \hbar \omega\left[ \frac{1}{\beta \hbar \omega}\right],$](img1529.png) |
(7.151) |
giving
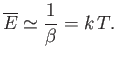 |
(7.152) |
Thus, the classical result, (7.139), holds whenever the thermal energy greatly exceeds the typical
spacing between quantum energy levels.
Consider the limit
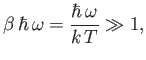 |
(7.153) |
in which the thermal energy is small compared to the separation between
the energy levels. In this limit,
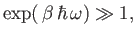 |
(7.154) |
and so
![$\displaystyle \overline{E} \simeq \hbar \omega \left[\frac{1}{2} + \exp(-\beta \hbar \omega)\right] \simeq \frac{1}{2} \hbar \omega.$](img1533.png) |
(7.155) |
Thus, if the thermal energy is much less than the spacing between quantum states then
the mean energy approaches that of the ground-state (the so-called zero-point
energy).
Clearly, the equipartition theorem is only valid in the former limit, where
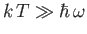 , and the oscillator possess sufficient thermal energy to explore many
of its possible quantum states.
, and the oscillator possess sufficient thermal energy to explore many
of its possible quantum states.



Next: Specific Heats
Up: Applications of Statistical Thermodynamics
Previous: Equipartition Theorem
Richard Fitzpatrick
2016-01-25
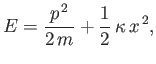
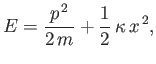
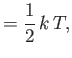
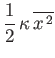
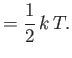
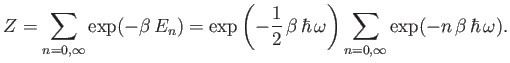
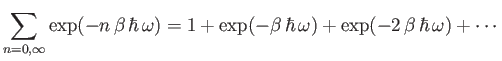
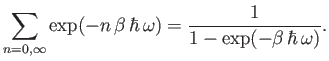
![$\displaystyle Z = \frac{ \exp[-(1/2) \beta \hbar \omega]}{1-\exp(-\beta \hbar \omega)},$](img1522.png)
![$\displaystyle \ln Z = - \frac{1}{2} \beta \hbar \omega -\ln \left[1- \exp(-\beta \hbar \omega)\right].$](img1523.png)
![$\displaystyle \overline{E} = - \frac{\partial}{\partial \beta} \ln Z = - \left[...
...(-\beta \hbar \omega) \hbar \omega} {1-\exp(-\beta \hbar \omega)}\right],$](img1524.png)
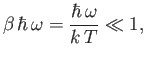
![$\displaystyle \overline{E} \simeq \hbar \omega\left[\frac{1}{2} + \frac{1}{\be...
...omega}\right] \simeq \hbar \omega\left[ \frac{1}{\beta \hbar \omega}\right],$](img1529.png)
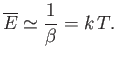
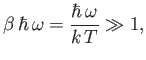
![$\displaystyle \overline{E} \simeq \hbar \omega \left[\frac{1}{2} + \exp(-\beta \hbar \omega)\right] \simeq \frac{1}{2} \hbar \omega.$](img1533.png)