

Next: Quantum Particles
Up: Wave-Particle Duality
Previous: Quantum Interference of Light
In this course, we are going to concentrate, almost exclusively, on the
behaviour of non-relativistic particles of non-zero mass (e.g., electrons). In the
absence of external forces, such particles, of mass 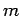 , energy
, energy 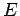 , and
momentum
, and
momentum 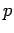 , move classically in a straight-line with velocity
, move classically in a straight-line with velocity
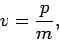 |
(71) |
and satisfy
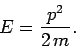 |
(72) |
Richard Fitzpatrick
2010-07-20