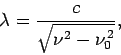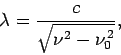

Next: Fundamentals of Quantum Mechanics
Up: Collapse of the Wave
Previous: Collapse of the Wave
- A He-Ne laser emits radiation of wavelength
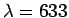 nm. How
many photons are emitted per second by a laser with a power of 1 mW?
What force does such laser exert on a body which completely absorbs its
radiation?
nm. How
many photons are emitted per second by a laser with a power of 1 mW?
What force does such laser exert on a body which completely absorbs its
radiation?
- The ionization energy of a hydrogen atom in its ground state is
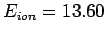 eV (1 eV is the energy acquired by an electron accelerated through a potential difference of 1 V). Calculate the frequency, wavelength,
and wavenumber of the electromagnetic radiation which will just ionize
the atom.
eV (1 eV is the energy acquired by an electron accelerated through a potential difference of 1 V). Calculate the frequency, wavelength,
and wavenumber of the electromagnetic radiation which will just ionize
the atom.
- The maximum energy of photoelectrons from aluminium is 2.3 eV
for radiation of wavelength
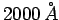 , and 0.90 eV for radiation of
wavelength
, and 0.90 eV for radiation of
wavelength 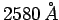 . Use this data to calculate Planck's constant,
and the work function of aluminium.
. Use this data to calculate Planck's constant,
and the work function of aluminium.
- Show that the de Broglie wavelength of an electron accelerated from rest across a potential difference
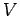 is given by
is given by
where 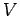 is measured in volts.
is measured in volts.
- If the atoms in a regular crystal are separated by
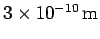 demonstrate that an accelerating
voltage of about
demonstrate that an accelerating
voltage of about 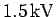 would be required to produce an electron diffraction pattern from the crystal.
would be required to produce an electron diffraction pattern from the crystal.
- The relationship between wavelength and frequency for electromagnetic waves in a waveguide is
where 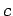 is the velocity of light in vacuum.
What are the group and phase velocities of such waves as functions of
is the velocity of light in vacuum.
What are the group and phase velocities of such waves as functions of 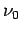 and
and 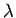 ?
?
- Nuclei, typically of size
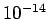 m, frequently emit electrons
with energies of 1-10 MeV. Use the uncertainty principle to show
that electrons of energy 1 MeV could not be contained in the nucleus
before the decay.
m, frequently emit electrons
with energies of 1-10 MeV. Use the uncertainty principle to show
that electrons of energy 1 MeV could not be contained in the nucleus
before the decay.
- A particle of mass
 has a wavefunction
has a wavefunction
where 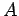 and
and 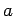 are positive real constants. For what potential
function
are positive real constants. For what potential
function 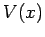 does
does 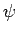 satisfy the Schrödinger equation?
satisfy the Schrödinger equation?


Next: Fundamentals of Quantum Mechanics
Up: Collapse of the Wave
Previous: Collapse of the Wave
Richard Fitzpatrick
2010-07-20