

Next: Photon Polarization
Up: Fundamental Concepts
Previous: Fundamental Concepts
The necessity for a departure from
classical physics is demonstrated by the following phenomena:
- Anomalous Atomic and Molecular Stability. According to classical
physics, an electron orbiting an atomic nucleus undergoes acceleration and should, therefore, lose energy via the continuous emission
of electromagnetic radiation, causing it to gradually spiral in towards the nucleus. Experimentally,
this is not observed to happen.
- Anomalously Low Atomic and Molecular Specific Heats. According to
the equipartition theorem of classical physics, each degree of freedom of
an atomic or molecular system should contribute
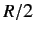 to its molar specific heat capacity, where
to its molar specific heat capacity, where 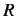 is the molar ideal gas constant.
In fact, only the translational, and some rotational, degrees of freedom seem
to contribute. The vibrational degrees of freedom appear to make no contribution
at all
(except at high temperatures). Incidentally, this fundamental
problem with classical physics was known and appreciated by the middle of the
nineteenth century. Stories that physicists at the start of the twentieth century thought that
classical physics explained everything, and that there was nothing left to
discover, are largely apocryphal (see Feynman, Volume I, Chapter 40).
is the molar ideal gas constant.
In fact, only the translational, and some rotational, degrees of freedom seem
to contribute. The vibrational degrees of freedom appear to make no contribution
at all
(except at high temperatures). Incidentally, this fundamental
problem with classical physics was known and appreciated by the middle of the
nineteenth century. Stories that physicists at the start of the twentieth century thought that
classical physics explained everything, and that there was nothing left to
discover, are largely apocryphal (see Feynman, Volume I, Chapter 40).
- Ultraviolet Catastrophe. According to classical physics, the equilibrium energy
density of an electromagnetic field contained within a vacuum cavity whose walls are held at a fixed temperature is infinite, due to a divergence of
energy carried by short wavelength modes. This divergence is called the ultraviolet catastrophe. Experimentally, there is no such
divergence, and the total energy density is finite.
- Wave-Particle Duality. Classical physics treats waves and
particles as completely distinct phenomena. However, various experiments (e.g., the interference of light, the photoelectric effect,
electron diffraction) demonstrate that waves sometimes act as if they
were streams of particles, and streams of particles sometimes act as if they
were waves. This behavior is completely inexplicable within the framework of
classical physics.


Next: Photon Polarization
Up: Fundamental Concepts
Previous: Fundamental Concepts
Richard Fitzpatrick
2013-04-08