


Next: Exercises
Up: Orbital Angular Momentum
Previous: Motion in Central Field
Energy Levels of Hydrogen Atom
Consider a hydrogen atom, for which the potential takes the specific form
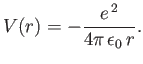 |
(4.119) |
The radial eigenfunction 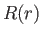 satisfies Equation (4.118), which can be written
satisfies Equation (4.118), which can be written
![$\displaystyle \left[\frac{\hbar^{\,2}}{2\,\mu} \left(-\frac{1}{r^{\,2}} \frac{d...
...l\,(l+1)}{r^{\,2}}\right) -\frac{e^{\,2}}{4\pi\,\epsilon_0\,r}- E\right] R = 0.$](img1166.png) |
(4.120) |
Here,
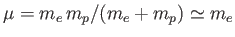 is the reduced mass, which takes into
account the fact that the electron (of mass
is the reduced mass, which takes into
account the fact that the electron (of mass 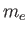 ) and the proton (of mass
) and the proton (of mass 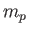 )
both orbit about a common centre of mass, which is equivalent to a particle of
mass
)
both orbit about a common centre of mass, which is equivalent to a particle of
mass 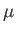 orbiting about a fixed point [50]. However, given that
orbiting about a fixed point [50]. However, given that
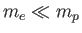 , we can
safely replace
, we can
safely replace 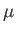 by
by 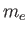 . (The correction involved in using
. (The correction involved in using 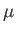 , rather than
, rather than 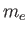 , in the analysis
is actually less than that involved in neglecting the electron's relativistic mass increase.) Let us write the product
, in the analysis
is actually less than that involved in neglecting the electron's relativistic mass increase.) Let us write the product 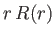 as the function
as the function 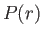 . The previous equation transforms to
. The previous equation transforms to
![$\displaystyle \frac{d^{\,2} P}{d r^{\,2}} - \frac{2\,m_e}{\hbar^{\,2}}\left[ \f...
...{\,2}}{2\,m_e \,r^{\,2}} - \frac{e^{\,2}}{4\pi \,\epsilon_0 \,r}-E\right] P =0,$](img1173.png) |
(4.121) |
which is the one-dimensional Schrödinger equation for a particle of
mass 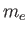 moving in the effective potential
moving in the effective potential
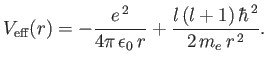 |
(4.122) |
The effective potential has a simple physical interpretation. The first part is the
attractive Coulomb potential, and the second part corresponds
to the repulsive centrifugal force.
Let
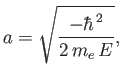 |
(4.123) |
and 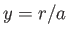 , with
, with
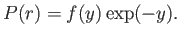 |
(4.124) |
Here, it is assumed that the energy eigenvalue 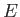 is negative.
Equation (4.121) transforms to
is negative.
Equation (4.121) transforms to
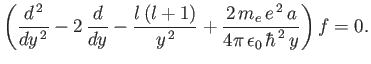 |
(4.125) |
Let us look for a power-law solution of the form
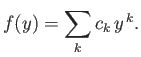 |
(4.126) |
Substituting this solution into Equation (4.125), we obtain
![$\displaystyle \sum_k c_k \left[ k\,(k-1)\,y^{\,k-2} - 2\,k\, y^{\,k-1} - l\,(l+...
...2\,m_e\, e^{\,2} \,a}{4\pi\, \epsilon_0 \,\hbar^{\,2}}\, y^{\,k-1} \right] = 0.$](img1180.png) |
(4.127) |
Equating the coefficients of 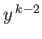 gives
gives
![$\displaystyle c_k\,[k\,(k-1) - l\,(l+1)] = c_{k-1} \left [2\,(k-1) - \frac{2\,m_e\, e^{\,2}\, a}{4\pi\, \epsilon_0\, \hbar^{\,2}}\right].$](img1182.png) |
(4.128) |
Now, the power-law series (4.126) must terminate at small 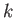 , at some positive
value of
, at some positive
value of 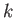 , otherwise
, otherwise 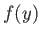 would diverge unphysically as
would diverge unphysically as
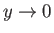 . This
is only possible if
. This
is only possible if
![$ [k_{\rm min} \,(k_{\rm min} -1) - l\,(l+1) ] =0$](img1185.png) , where
the first term in the series is
, where
the first term in the series is
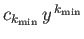 . There
are two possibilities:
. There
are two possibilities:
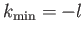 or
or
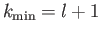 . The
former predicts unphysical divergence of the wavefunction at
. The
former predicts unphysical divergence of the wavefunction at 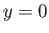 .
Thus, we conclude that
.
Thus, we conclude that
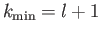 , and that the first term in the series is
, and that the first term in the series is
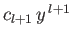 . Note that for an
. Note that for an 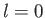 state
there is a finite probability of finding the electron at the nucleus,
whereas for an
state
there is a finite probability of finding the electron at the nucleus,
whereas for an 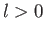 state there is zero probability of finding
the electron at the nucleus (i.e.,
state there is zero probability of finding
the electron at the nucleus (i.e.,
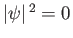 at
at  , except when
, except when
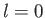 ). Furthermore, it is only possible to obtain sensible behavior of the
wavefunction as
). Furthermore, it is only possible to obtain sensible behavior of the
wavefunction as
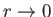 if
if  is an integer.
is an integer.
For large values of 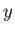 , the ratio of successive terms in the series
(4.126) is
, the ratio of successive terms in the series
(4.126) is
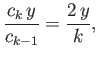 |
(4.129) |
according to Equation (4.128). This is the same as the ratio of
successive terms in the series
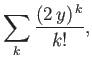 |
(4.130) |
which converges to
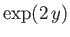 . We conclude that
. We conclude that
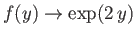 as
as
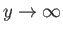 . It follows from Equation (4.124) that
. It follows from Equation (4.124) that
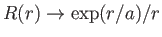 as
as
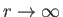 . This does not correspond to
physically acceptable behavior of the wavefunction, because
. This does not correspond to
physically acceptable behavior of the wavefunction, because
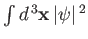 must be finite. The only way in which we can avoid this unphysical
behavior is if the series (4.126) terminates at some maximum value of
must be finite. The only way in which we can avoid this unphysical
behavior is if the series (4.126) terminates at some maximum value of 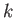 .
According to the recursion relation (4.128), this is only possible
if
.
According to the recursion relation (4.128), this is only possible
if
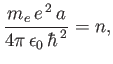 |
(4.131) |
where the last term in the series is
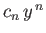 . It follows from Equation (4.123)
that the energy eigenvalues are quantized, and can only take the values
. It follows from Equation (4.123)
that the energy eigenvalues are quantized, and can only take the values
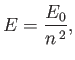 |
(4.132) |
where
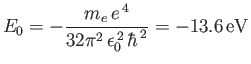 |
(4.133) |
is the energy of the ground state (i.e., the lowest energy state).
Here,  is a positive integer that must exceed the quantum number
is a positive integer that must exceed the quantum number  ,
otherwise there would be no terms in the series (4.126).
Expression (4.132) is known as the Bohr formula, because it was derived
heuristically by Niels Bohr in 1913 [10].
,
otherwise there would be no terms in the series (4.126).
Expression (4.132) is known as the Bohr formula, because it was derived
heuristically by Niels Bohr in 1913 [10].
In summary, the properly normalized wavefunction of a hydrogen atom is written
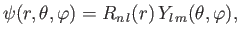 |
(4.134) |
where
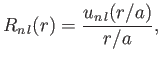 |
(4.135) |
and
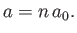 |
(4.136) |
Here,
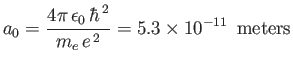 |
(4.137) |
is the Bohr radius, and
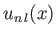 is a well-behaved solution of the differential equation
is a well-behaved solution of the differential equation
![$\displaystyle \left[\frac{d^{\,2}}{dx^{\,2}}-\frac{l\,(l+1)}{x^{\,2}} + \frac{2\,n}{x} - 1\right] u_{n\,l} = 0$](img1210.png) |
(4.138) |
that satisfies the normalization constraint
![$\displaystyle a^{\,3}\int_0^\infty dx\,[u_{n\,l}(x)]^{\,2}= \int_0^\infty dr\,r^{\,2}\left[R_{nl}(r)\right]^{\,2} = 1.$](img1211.png) |
(4.139) |
Finally, the
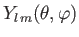 are spherical harmonics. The restrictions on the quantum numbers
are
are spherical harmonics. The restrictions on the quantum numbers
are
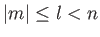 , where
, where  is a positive integer,
is a positive integer,  a non-negative integer, and
a non-negative integer, and 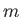 an integer. Incidentally, the quantum numbers
an integer. Incidentally, the quantum numbers  ,
,  , and
, and 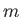 are conventionally referred to as the principle quantum number, the azimuthal quantum number,
and the magnetic quantum number, respectively.
are conventionally referred to as the principle quantum number, the azimuthal quantum number,
and the magnetic quantum number, respectively.
The ground state of hydrogen corresponds to  . The only permissible values
of the other quantum numbers are
. The only permissible values
of the other quantum numbers are 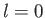 and
and 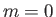 . Thus, the ground state is
a spherically symmetric, zero angular momentum, state. The next energy level corresponds to
. Thus, the ground state is
a spherically symmetric, zero angular momentum, state. The next energy level corresponds to  . The other quantum numbers are
allowed to take the values
. The other quantum numbers are
allowed to take the values 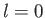 ,
, 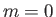 or
or 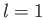 ,
,
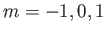 . Thus, there are
. Thus, there are
 states with non-zero angular momentum. Note that the energy levels given
in Equation (4.132) are independent of the quantum number
states with non-zero angular momentum. Note that the energy levels given
in Equation (4.132) are independent of the quantum number  , despite the fact that
, despite the fact that
 appears in the radial eigenfunction equation (4.138). This is a special
property of a
appears in the radial eigenfunction equation (4.138). This is a special
property of a  Coulomb potential.
Coulomb potential.
In addition to the quantized negative energy states of the
hydrogen atom, which we have just found, there
is also a continuum of unbound positive energy states.



Next: Exercises
Up: Orbital Angular Momentum
Previous: Motion in Central Field
Richard Fitzpatrick
2016-01-22
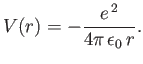
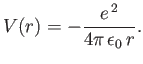
![$\displaystyle \left[\frac{\hbar^{\,2}}{2\,\mu} \left(-\frac{1}{r^{\,2}} \frac{d...
...l\,(l+1)}{r^{\,2}}\right) -\frac{e^{\,2}}{4\pi\,\epsilon_0\,r}- E\right] R = 0.$](img1166.png)
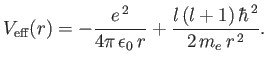
![$\displaystyle \sum_k c_k \left[ k\,(k-1)\,y^{\,k-2} - 2\,k\, y^{\,k-1} - l\,(l+...
...2\,m_e\, e^{\,2} \,a}{4\pi\, \epsilon_0 \,\hbar^{\,2}}\, y^{\,k-1} \right] = 0.$](img1180.png)
![]() , the ratio of successive terms in the series
(4.126) is
, the ratio of successive terms in the series
(4.126) is
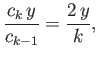
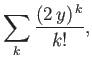
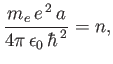
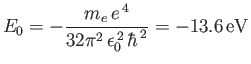
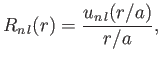
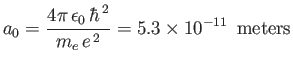
![$\displaystyle a^{\,3}\int_0^\infty dx\,[u_{n\,l}(x)]^{\,2}= \int_0^\infty dr\,r^{\,2}\left[R_{nl}(r)\right]^{\,2} = 1.$](img1211.png)
![]() . The only permissible values
of the other quantum numbers are
. The only permissible values
of the other quantum numbers are ![]() and
and ![]() . Thus, the ground state is
a spherically symmetric, zero angular momentum, state. The next energy level corresponds to
. Thus, the ground state is
a spherically symmetric, zero angular momentum, state. The next energy level corresponds to ![]() . The other quantum numbers are
allowed to take the values
. The other quantum numbers are
allowed to take the values ![]() ,
, ![]() or
or ![]() ,
,
![]() . Thus, there are
. Thus, there are
![]() states with non-zero angular momentum. Note that the energy levels given
in Equation (4.132) are independent of the quantum number
states with non-zero angular momentum. Note that the energy levels given
in Equation (4.132) are independent of the quantum number ![]() , despite the fact that
, despite the fact that
![]() appears in the radial eigenfunction equation (4.138). This is a special
property of a
appears in the radial eigenfunction equation (4.138). This is a special
property of a ![]() Coulomb potential.
Coulomb potential.